Mercury is found in almost all oil and gas reservoirs, principally in the elemental form at varying concentrations. Mercury present in produced hydrocarbon fluids deposits onto the internal process infrastructure via a number of mechanisms, including chemisorption and adsorption. The primary mechanism of deposition observed by Qa3 is through reaction with iron sulphide to form mercury sulphide, demonstrated by the case study below.
In 2018, Qa3 undertook initial trace contaminant analysis in gas during well testing operations of a new gas field in the Caribbean. Samples were taken offshore at the wellhead and onshore at the receiving facility once the fluid had transited a ~40 km subsea pipeline. Quantification of H2S identified there was a 50% decrease from offshore to onshore, most likely due to reaction of H2S with iron oxides or other corrosion products on the internal surfaces of the carbon steel subsea pipeline. In contrast, no loss of mercury was observed between the wellhead platform and the receiving facility (data generated offshore and onshore was the same within experimental uncertainty). See Figure below.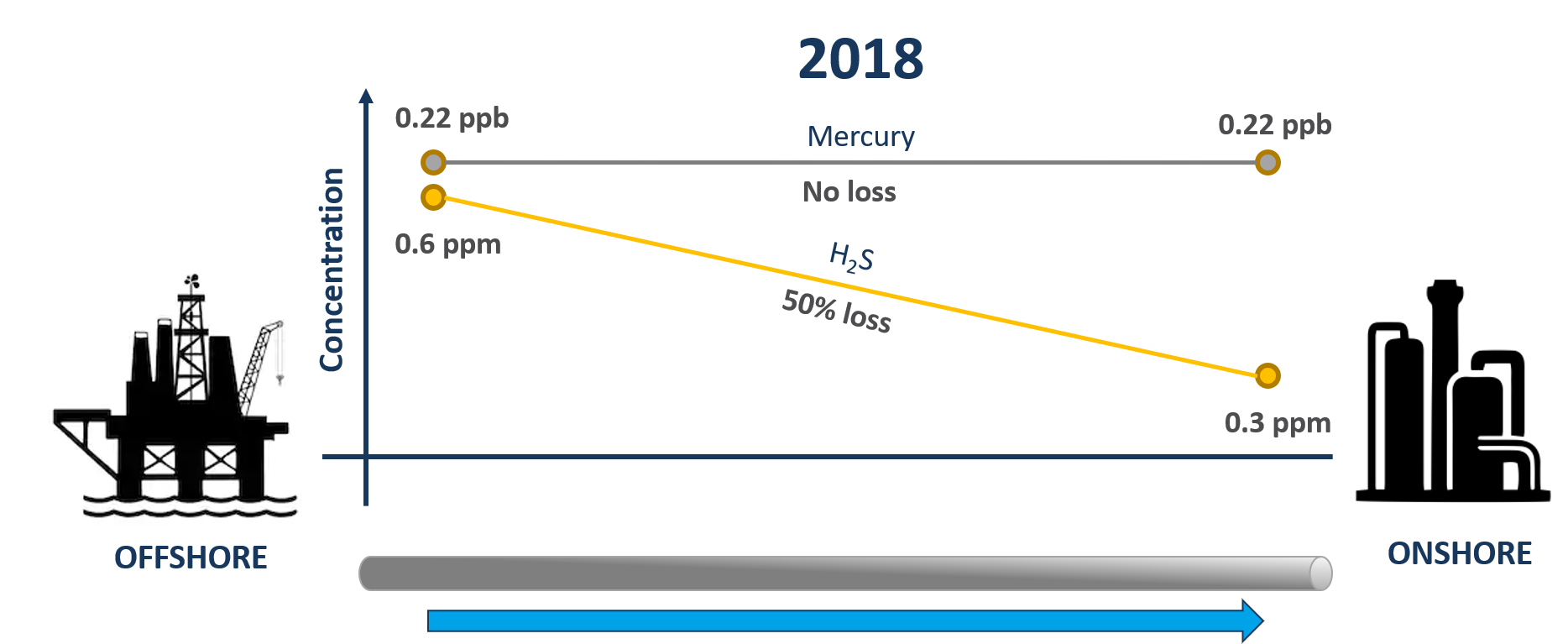
Qa3 were expecting to see some of the mercury removed by the pipeline during the initial survey as it is known elemental mercury can adsorb into cracks and fissures on the internal surface of the pipeline and and corrosion scale (1) were surprised to observe all mercury transiting though the pipeline. Qa3 were subsequently informed that the subsea pipeline had been filled with seawater prior to being brought into service which resulted in a biofilm forming on the internal surface. It is theorised that this initial biofilm hindered the adsorption of elemental mercury. This biofilm may have also impacted the rate of iron sulphide formation.
In 2022, Qa3 were engaged to undertake a similar trace contaminant survey on the same gas field. Interestingly, the quantification of H2S demonstrated no further loss from offshore to onshore suggesting that a saturated sulphide scale layer had formed across the entire length of internal surface of the pipeline meaning that there were no more active sites remaining with which H2S could react. Contrastingly, mercury quantification demonstrated a loss of 69% from offshore to onshore, indicative of reaction of elemental mercury with the iron sulphide scale layer to form mercury sulphide which remains bound within the scale layer. See Figure below. This mechanism of reaction is similar to that employed in many commercial mercury removal units, where a metal sulphide is used to capture elemental mercury as mercury sulphide.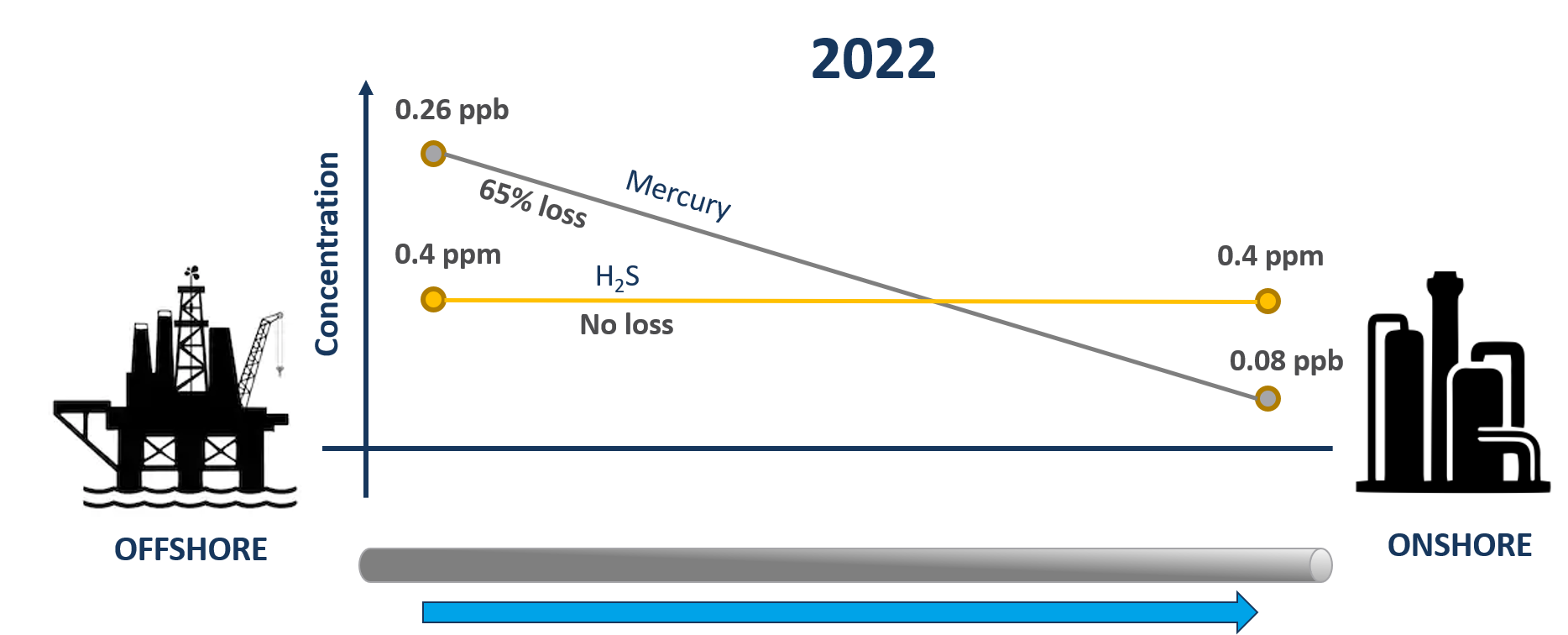
The precise dynamics of these reactions will be dependent on a number of factors including but not limited to, scale density, steel grade, extent of internal corrosion, temperature, water concentration, flow rates, oxygen ingress etc.
Over the lifetime of facilities, the accumulation of this mercury sulphide layer can equate to pipeline scales containing % concentrations of mercury. Thus, aged facilities and infrastructure that have reached the end of their operational life and are selected for either recycling or abandonment, may pose a serious risk to health and the environment if the decommissioning process is not managed correctly. Follow this link to learn more: mercury in oil and gas infrastructure for decommissioning.
Are you producing mercury in hydrocarbon fluids offshore but not seeing it arrive at your onshore facility?
Do you want to gain a better understanding of the mercury profile across your assets?
Have you taken into account the implications of the presence scale that could contain % concentrations of mercury when considering pipeline or asset end of life options?
For more information on this case study or for general support with understanding the impact of mercury across your process, please do not hesitate to get in touch.
The data in this case study has been adjusted slightly to protect the confidentiality of the client’s data, although the % reduction of mercury and hydrogen sulphide remains the same.
(1) (1) Lhiam Paton, Peter Crafts, David Clases, Thomas Lindsay, Andreas Zimmer, Henrik Siboni, Raquel Gonzalez de Vega, J¨org Feldmann. (2023) The impact of corrosion on the adsorption of gaseous Hg0 onto the surface of steels: Implications for decommissioning in the oil and gas industry; Journal of Hazardous Materials 458 (2023) 131975.